Unleashing the Power of the Immune System Against Cancer
Prof. Aécio D’Silva, Ph.D
AquaUniversity
A groundbreaking immunotherapy, CAR-T cell therapy, is transforming the landscape of cancer treatment, offering new hope and improved outcomes for patients battling various forms of cancer.
In recent years, significant advancements have been made in the field of cancer treatment, revolutionizing the way we approach this devastating disease. One such breakthrough is CAR-T cell therapy, which stands for chimeric antigen receptor T-cell therapy. This innovative treatment utilizes the patient’s immune system to target and destroy cancer cells. In this blog, we will delve into the intricacies of this treatment, explore its remarkable advances, and shed light on the potential benefits it holds for cancer patients worldwide.
Understanding CAR-T Cell Therapy
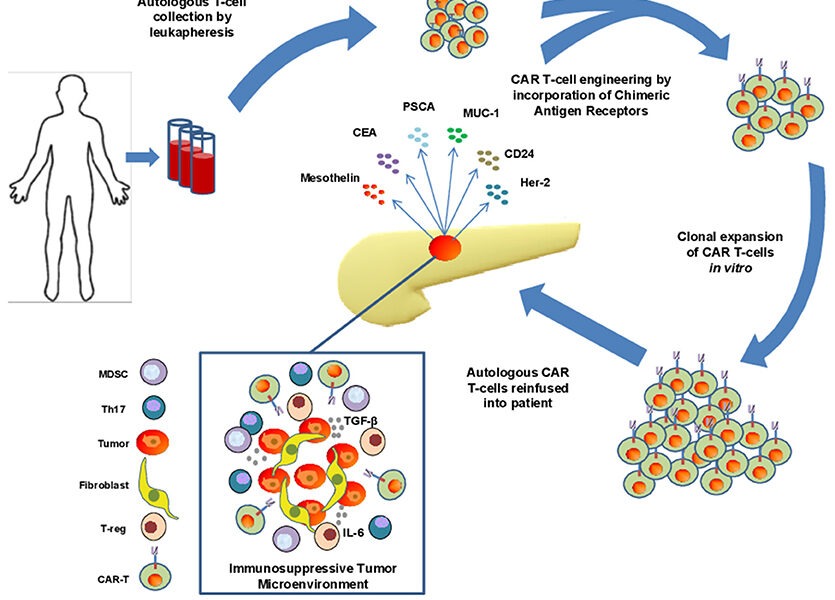
CAR-T cell therapy is a personalized form of immunotherapy that harnesses the power of a patient’s own immune cells to combat cancer. The therapy involves extracting a patient’s T cells, a type of white blood cell crucial for immune response, and modifying them in a laboratory setting. The modification includes introducing a chimeric antigen receptor (CAR) into the T cells, which enables them to recognize and bind to specific antigens present on cancer cells.
Once the T cells have been genetically modified to express the CAR, they are multiplied.
CAR-T cells then navigate through the body, hunting down cancer cells that bear the targeted antigen. When the CAR-T cells recognize their designated antigen in cancer cells, they initiate a powerful immune response, ultimately leading to the destruction of malignant cells.
Advances in CAR-T Cell Therapy
Since its inception, this innovative treatment has achieved remarkable progress, leading to enhanced treatment outcomes for patients battling a range of cancers. Notably, the FDA approval of two CAR-T cell therapies, Kymriah and Yescarta, has opened new avenues for the treatment of acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma, respectively. Ongoing clinical trials and research are exploring the potential of this treatment in treating various other malignancies, including solid tumors.
Furthermore, advancements in CAR-T cell engineering techniques have improved the effectiveness and safety of the therapy. Scientists are continuously refining the design of CAR receptors, optimizing their binding affinity and specificity for cancer cells while minimizing off-target effects. These developments contribute to minimizing potential adverse events and expanding the scope of CAR-T cell therapy to a broader patient population.
Potential Benefits of CAR-T Cell Therapy
The potential benefits of CAR-T cell therapy are immense and hold promise for transforming the lives of cancer patients worldwide. Firstly, this innovative therapy has demonstrated remarkable efficacy, often achieving deep and durable remissions in patients who have exhausted conventional treatment options. It offers hope for individuals facing aggressive cancers that were previously considered incurable.
Secondly, CAR-T cell therapy is highly specific, as it targets only cancer cells expressing the selected antigen. This specificity minimizes damage to healthy cells and reduces the side effects commonly associated with traditional cancer treatments like chemotherapy and radiation therapy. By sparing healthy tissues, CAR-T cell therapy can potentially improve patients’ quality of life during and after treatment.
Challenges and Future Directions
While CAR-T cell therapy holds immense promise in revolutionizing cancer treatment, there are still challenges that need to be overcome for its wider adoption and accessibility. The high cost associated with manufacturing and administration, along with potential side effects, presents barriers to its widespread use. Additionally, the limited availability of clinical trials for different cancer types restricts its application in a broader patient population.
However, ongoing research and clinical trials are actively addressing these challenges. Efforts are being made to optimize manufacturing processes, reducing costs and improving scalability. Moreover, advancements in CAR-T cell engineering techniques are focused on enhancing safety profiles and minimizing off-target effects. This continuous progress is paving the way for broader accessibility and improved outcomes for cancer patients.
As we move forward, it is crucial to continue investing in research, development, and clinical trials to further refine CAR-T cell therapy. Collaborations between researchers, healthcare professionals, and pharmaceutical companies are vital in expanding its applications to various cancer types and exploring combination therapies for even greater efficacy.
To conclude, the potential benefits of CAR-T cell therapy are tremendous, and as we overcome the challenges, this groundbreaking treatment has the potential to become a standard of care in the fight against cancer. By harnessing the power of the immune system, this amazing treatment brings new hope to patients and their families, offering the possibility of long-lasting remissions and improved quality of life.
References:
- Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric Antigen Receptor T-Cell Therapy — Assessment and Management of Toxicities. Nat Rev Clin Oncol. 2018;15(1):47-62.
- June CH, Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med. 2018;379(1):64-73.
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439-448.
- Grupp SA, Maude SL, Shaw PA, et al. Durable Remissions in Children with Relapsed/Refractory ALL Treated with T Cells Engineered with a CD19-Targeted Chimeric Antigen Receptor (CTL019). Blood. 2015;126(23):681.
- Abudayyeh OO, Gootenberg JS, Essletzbichler P, et al. RNA Targeting with CRISPR-Cas13. Nature. 2017;550(7675):280-284.