Contents
- 1 Electrolyzers – Why They’re Essential to the Future of Green Hydrogen Production
- 2 By Prof Aecio D’Silva
- 3 AquaUniversity.com
- 4 What is an electrolyzer and how does it work?
- 5 Are there various kinds of electrolyzers?
- 6 What is the status of the electrolyzer application?
- 7 Advancements in electrolyzers for green hydrogen production
Electrolyzers – Why They’re Essential to the Future of Green Hydrogen Production
By Prof Aecio D’Silva
AquaUniversity.com
Electrolyzers are a key component of many clean energy systems today. They are used to generate hydrogen for fuel cells, which are clean and efficient devices that convert chemical energy into electrical energy. Electrolyzers can be broken down into alkaline, PEM, and SOEC types. Each type has its own strengths and weaknesses, but all three are important to the development of clean energy systems.
In this article, we’ll take a closer look at how electrolyzers work and why they’re essential to our future.
(Image credit: Greg Stewart, SLAC National Accelerator Laboratory)
What is an electrolyzer and how does it work?
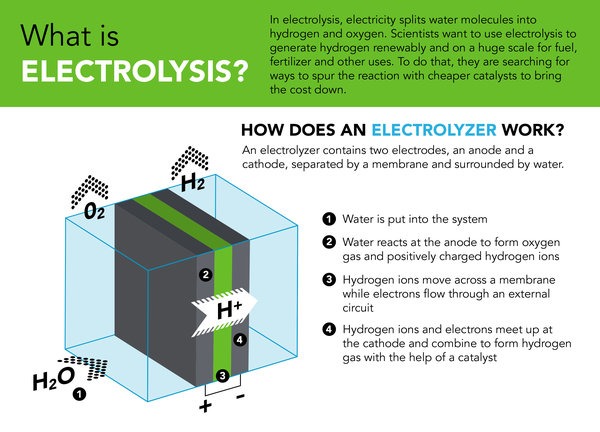
An electrolyzer is a device that splits water into hydrogen and oxygen by passing an electric current through it. The process of electrolyzing water to produce hydrogen gas was first discovered by Sir William Grove in 1839. Since then, several distinct types of electrolyzers have been developed to meet various needs.
It’s made up of two electrodes: one positive and one negative. When you apply electricity to the electrolyzer, hydrogen gas will be released at one electrode (the cathode), while oxygen gas will be released at the other electrode (the anode). This process is called electrolysis.
Electrolyzers are used in many different applications, including industrial processes, chemical production, and fuel production. The hydrogen produced by electrolyzers is often used as an alternative fuel source for vehicles or for generating electricity.
Are there various kinds of electrolyzers?
Yes! There are three main types of electrolyzers: alkaline, proton exchange membrane (PEM), and solid oxide electrolyzers (SOEC). Each type has its unique characteristics, applications, advantages, and disadvantages, but they all produce hydrogen fuel at relatively low temperatures and pressures.
Alkaline Electrolyzers:
These are the most common types of electrolyzers today. They also require a liquid electrolyte solution such as potassium hydroxide (KOH) or sodium hydroxide (NAOH), and water.
Alkaline electrolyzers use an alkali metal ion solution (such as potassium hydroxide) and carbon electrodes to produce hydrogen and oxygen gases from water.
They’re not as efficient because they require a lot of energy to function properly—and often require extra steps like adding heat exchangers or cooling systems to improve efficiency.
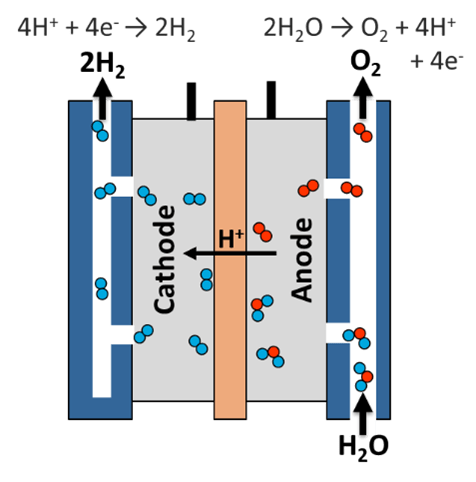
Hydrogen is produced by separating it from oxygen in a series of “cells.” Each cell consists of an anode, cathode, and membrane—adding more cells to the stack increases both the amount of hydrogen generated as well as the amount of oxygen created.
Applying a voltage across the stack of cells causes the hydroxide ions (OH-) to move from cathodes to anodes, with hydrogen gas bubbles forming at cathode surfaces and oxygen gas at anode ones.
Proton Exchange Membrane (PEM) Electrolyzers:
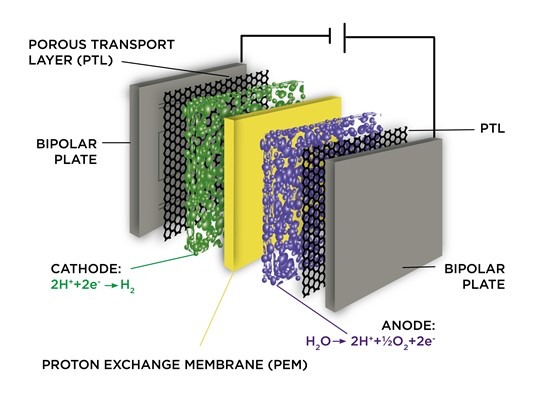
PEMs are more efficient than alkaline systems because they can operate at higher temperatures without using platinum catalysts—they only need a platinum-based membrane to separate hydrogen from oxygen gas molecules. Proton exchange membrane (PEM) electrolyzers use an ionic liquid solution and produce hydrogen at room temperature.
PEM electrolyzers use a solid polymer membrane through which hydrogen protons pass to form H2 gas. When current is applied to the cell stack, water splits into its components of oxygen and hydrogen at the anode side and then the H2 passes through the Membrane to the cathode
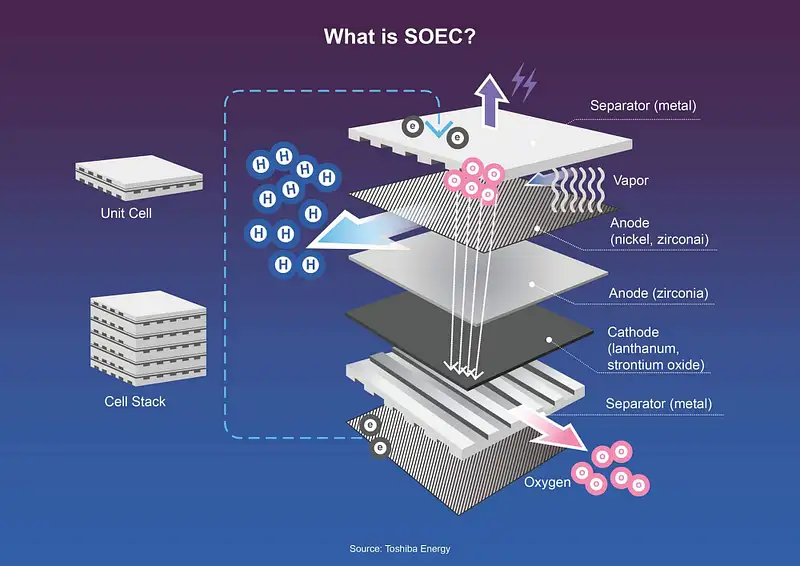
Solid Oxide Electrolyzers (SOEC):
(SOEC) use solid oxide ceramic electrolyte cells to split water into hydrogen and oxygen. These are the most efficient electrolyzers currently available, but they’re also expensive. (SOEC) use an oxide material as a catalyst to break down water into hydrogen and oxygen gases.
The process requires elevated temperatures and pressure, which make it more expensive than other methods; however, it has the highest efficiency of any electrolyzer design.
How to Select Electrolyzers
Electrolyzers can be used to produce hydrogen from a variety of feedstocks, including water and natural gas. They’re available in a range of sizes, power capacities, and fuel types. The main factors to consider when choosing an electrolyzer include efficiency (how much hydrogen it produces), how much you need, operating pressure, and cost. Portable systems have a lower initial investment than stationary ones, but they’re less efficient and aren’t as easy to scale up.
For example, for a small-scale research project or demonstration, the simplest and least expensive electrolyzer may be sufficient. However, if you’re considering a commercial application that requires high-quality hydrogen from renewable sources at low costs and rapid turn-around times then investing in a solid oxide electrolyzer or flow cell is probably your best option.
What is the status of the electrolyzer application?
The use of electrolyzers has become a common method to convert water into hydrogen and oxygen. The demand for clean energy is increasing rapidly, which makes it necessary to find cleaner ways to produce hydrogen. Electrolyzers are expected to play a vital role in this regard.
Electrolyzers are currently being used in a wide range of applications. The most common uses include water treatment and fuel cell production. Water treatment also includes the removal of CO2 from flue gases generated by fossil fuel power plants. For example, a Norsk Hydro facility in Norway is using electrolyzers to produce hydrogen from renewable hydroelectric sources to power fuel cells that provide clean drinking water for local communities.
Electrolyzers are currently being used in a variety of applications, including:
-Producing hydrogen from water (and other chemicals) for use in fuel cells.
-Water treatment.
-Industrial chemical production and purification processes.
-Ammonia production requires vast amounts of hydrogen, which means that renewable energy electricity can now be utilized for producing hydrogen to supply green ammonia fertilizer production.
-Production of green hydrogen from renewable energy sources
-Hydrogen fueling stations that provide fuel to hydrogen vehicles
-Production of chemicals such as chlorine and caustic soda from water and salt as of 2014, electrolyzers are being used to produce green hydrogen from renewable energy sources. They’re also being used in the semiconductor industry and by other manufacturers who need high-purity hydrogen for their processes.
Advancements in electrolyzers for green hydrogen production
Electrolyzer technology is improving, and the market for hydrogen-powered vehicles is growing. Hydrogen fuel cells are being used in cars, buses, trucks, and trains.
Electrolyzers function as the backbone of green hydrogen production from solar, wind, or other forms of renewable energy. They produce pure green hydrogen by splitting water into hydrogen and oxygen. Hydrogen can be used for energy or stored for later use.
The electrolysis of water is not a recent technology nor is the separation of hydrogen and oxygen. Electrolysis works in a manner opposite to the way batteries produce electricity: instead of generating current, electrolysis uses electrical energy to break water molecules into their base components—hydrogen and oxygen gas.
But it has taken decades to perfect and bring costs down to what they are today. One of the major drawbacks was the need for platinum or another precious metal as the catalyst until a research team at the US Department of Energy’s SLAC National Accelerator Laboratory and Stanford University developed an approach using a combination of titanium, cobalt, and nickel that could increase efficiency while reducing costs. Reactions that produce hydrogen gas and oxygen take place on different electrodes with different precious metal catalysts.
In this case, the research Hydrogen team replaced the platinum catalyst on the hydrogen-generating side with a catalyst consisting of cobalt phosphide nanoparticles deposited on carbon to form a fine black powder, which was produced by the researchers at SLAC and Stanford. Like other catalysts, it brings other chemicals together and encourages them to react.
Numerous studies have shown that electrolyzers are currently one of the most efficient methods for producing hydrogen from electricity when compared to other technologies such as steam methane reforming.
If you have any involvement in renewable energy issues, green hydrogen being one of the most important recent technologies, then understanding what an electrolyzer is will make all the difference in your decisions.